Autoimmune
Autoimmunity is a form of chronic inflammation where the body’s immune system mistakenly attacks its own cells. There are over 80 different autoimmune diseases affecting approximately 5% of the population. The most common autoimmune diseases include rheumatoid arthritis, multiple sclerosis, type 1 diabetes mellitus and psoriasis. The Nyrada Autoimmune Disease Program involves the development of an inhibitor for the treatment of autoimmune diseases such as psoriasis, ankylosing spondylitis and rheumatoid arthritis.
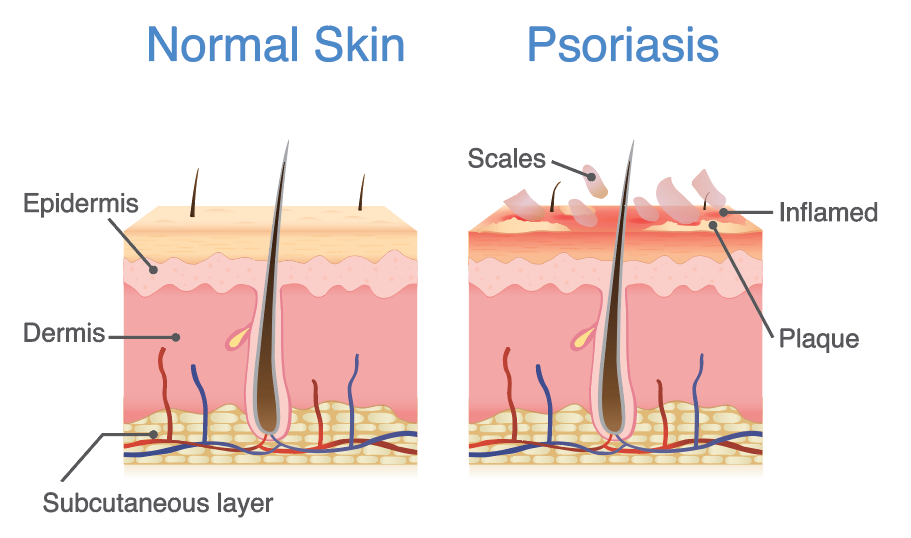
Psoriasis is a common, autoimmune, skin condition affecting at least 100 million people worldwide. Of the 23.5 million Americans suffering from autoimmune diseases, 7.5 million Americans suffer from psoriasis. The condition is characterised by an increase in the life cycle of skin cells, manifesting as scales and red patches of build-up skin cells that are often itchy, uncomfortable and impact severely on a person’s quality of life. A multitude of health issues affect psoriasis patients, including increased prevalence of metabolic syndromes such as hyperlipidaemia, hypertension, coronary artery disease and type 2 diabetes, indicating a need to treat psoriasis early before the onset of debilitating metabolic syndromes. Patients also often feel stigmatized and experience depression, which impacts their quality of life, productivity and overall well-being.
Psoriasis is most effectively treated by monoclonal antibodies- targeting either IL-23 or TNF-α. These medications include Humira (AbbVie), Tremfya (Janssen) and Skyrizi (AbbVie), and need to be injected every eight to twelve weeks. Injections must be stored in a climate-controlled environment, making them unsuitable to travel with, and the injections themselves are quite uncomfortable for patients. Further, the prohibitively high cost of monoclonal therapies makes them inaccessible to most psoriasis patients. Skyrizi comes with a first-year price tag of US$88,500 and a yearly maintenance-dose price of US$59,000. Humira, which comes with a cost of $1484 per treatment, had global sales of approximately US$19.9 billion in 2018. Therefore, although monoclonal antibodies are highly effective, their price limits them from becoming the standard of care for the treatment of psoriasis.
Nyrada is developing an IRAK4 inhibitor, to be taken orally or as a cream for the treatment of psoriasis. The IRAK4 protein is upstream in the inflammatory cascade, with inhibition of IRAK4 expected to reduce the downstream production of IL-23 and other proinflammatory molecules. Although there are currently no FDA-approved IRAK4 inhibitors, there are several in development including Pfizer who are in Phase II studies for rheumatoid arthritis. Therefore, Nyrada is confident that targeting these kinases will deliver a safe, cost-effective therapeutic treatment for psoriasis sufferers.
The precise binding mechanism of action of the Company’s novel IRAK4 inhibitor to its IRAK4 protein target has been determined by X-ray Crystallography. This has allowed for more precise molecular modelling, which has resulted in the development of more potent drug analogues. Compound optimisation is anticipated to continue over the next 18 months. The primary autoimmune clinical indication being sought is psoriasis.




